
#Electronegativity periodic table full#
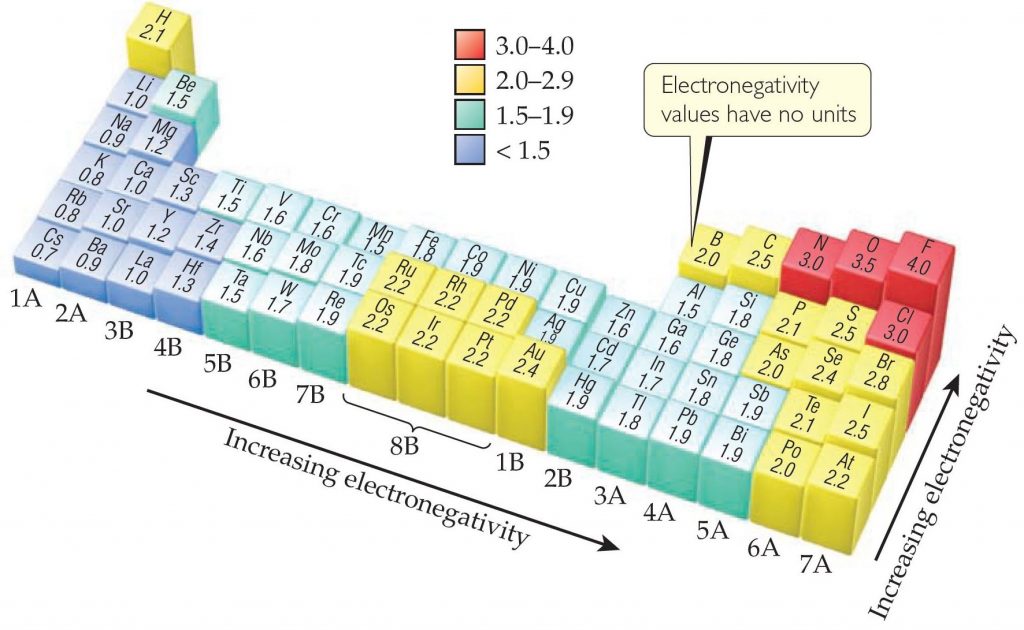
Noble gases are excluded from the electronegativity list because these atoms usually do not share electrons with other atoms since they have a full valence shell.Metals tend to be less electronegative elements, and the group 1 metals have the lowest electronegativities.


The greater the difference in electronegativity, the more polarized the electron distribution and the larger the partial charges of the atoms. Electrons in a polar covalent bond are shifted toward the more electronegative atom thus, the more electronegative atom is the one with the partial negative charge. The more strongly an atom attracts the electrons in its bonds, the larger its electronegativity. To account for this difference, Pauling suggested that the bond must have an ionic character, which is determined by the concept of electronegativity.Įlectronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself.Įlectronegativity determines how the shared electrons are distributed between the two atoms in a bond. However, the experimentally obtained bond energy of HF is 565 kJ/mol, which is much higher than the predicted value. Based on the values, he proposed that the energy required to break a bond will be the average of bond energies of H 2 (436 kJ/mol) and F 2 (155 kJ/mol), i.e., 296 kJ/mol. Pauling investigated the energies required to break bonds in heteronuclear molecules such as hydrogen and fluoride. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity.Įlectronegativity values of the elements were proposed by one of the most famous chemists of the twentieth century: Linus Pauling. Francium, on the other hand, is the least electronegative element with the electronegativity value of 0.7.Įlectronegativity has no unit it cannot be determined experimentally. In the periodic table, electronegativity values increase from left to right - metals are less electronegative compared to nonmetals, with exception to transition metals.Īdditionally, the electronegativity values decrease down the column and with increasing atomic size, because atoms are less able to attract electrons to themselves.įluorine, the most electronegative element, has the arbitrarily assigned electronegativity value of 3.98. He established an electronegativity scale based on thermochemical data, which helps predict bond types.Įlectronegativity is associated with the ionization energy and electron affinity of the atoms. The American Chemist Linus Pauling studied energies required to break bonds in molecules such as diatomic chlorine or hydrogen. Thus, in addition to nonpolar covalent or ionic bonds, polar covalent bonds are found across a large variety of compounds. The greater the difference in electronegativity between two atoms, the more polar the bond will be. The electron density is higher on the chlorine than on the hydrogen atom forming a polar covalent bond. In an ionic bond, electrons are transferred from metals to nonmetals, while in HCl, the electrons are unequally shared. This, however, does not make the bond ionic. Chlorine is thus said to be more electronegative than hydrogen, attracting the shared electrons towards itself, while resisting the removal of its own electrons. The ability of an atom to attract electrons towards itself is called electronegativity. For instance, if gaseous nitrogen is placed in an electric field, it will orient equally between the poles.īut when gaseous hydrogen chloride, a neutral molecule, is placed in an electric field, the hydrogen orients towards the cathode and chlorine towards the anode, indicating that hydrogen has a partial positive charge and chlorine has a partial negative charge. The Lewis model depicts all covalent bonds as equally shared electrons however, this is not always the case. But are these electrons shared equally among both atoms, or does one atom attract the electrons more than the other? Nonmetals form covalent bonds by sharing electrons.


 0 kommentar(er)
0 kommentar(er)
